Most people assume a pharmaceutical patent lasts 20 years. That’s what the law says. But if you look at when a drug actually hits the market, the real clock starts ticking much later-and it runs out far sooner than you’d expect.
Why the 20-Year Patent Doesn’t Mean 20 Years of Sales
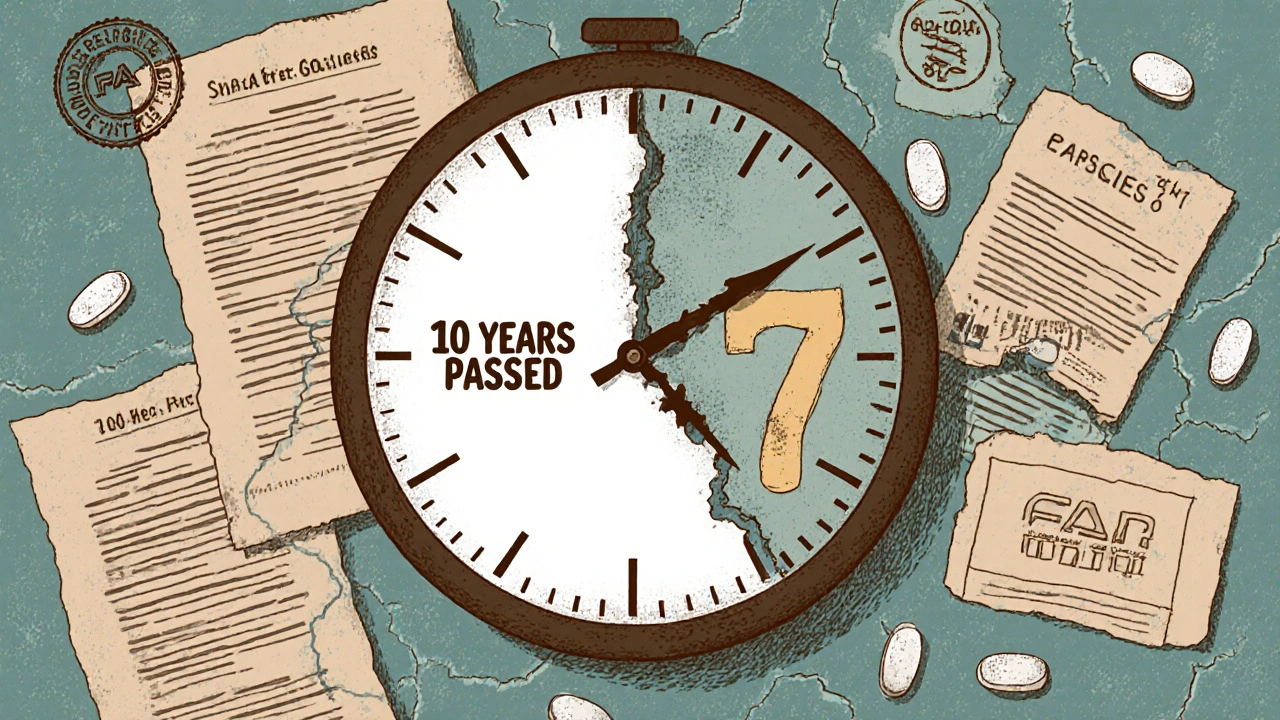
A drug patent is filed as soon as a company identifies a promising molecule. That’s often 10 to 15 years before the FDA approves it for sale. By the time the drug reaches pharmacies, half the patent clock has already run out.
Here’s how it breaks down: The U.S. Patent and Trademark Office grants patents for 20 years from the filing date. But clinical trials alone take 6 to 10 years. Add in the FDA review process-another 1 to 2 years-and you’re left with just 7 to 10 years of actual market exclusivity. That’s not a glitch. It’s the system.
Take a typical new cancer drug. Scientists file the patent in 2010. The drug doesn’t get FDA approval until 2020. That leaves only 10 years before generics can enter. But even that 10-year window isn’t guaranteed. Companies spend millions fighting off competitors with legal tricks, regulatory loopholes, and secondary patents. The real window for profit? Often closer to 7 years.
The Hatch-Waxman Act: A Compromise That Didn’t Last
In 1984, Congress passed the Hatch-Waxman Act to balance two goals: reward innovation and get cheap generics to patients fast. It gave drugmakers a lifeline: Patent Term Extension (PTE). If a drug spent years stuck in regulatory limbo, the company could get up to five extra years of patent life.
But there’s a catch. The extension can’t push total market exclusivity past 14 years from FDA approval. So even if a drug took 12 years to get approved, the patent can’t be extended beyond 14 years of sales time. That’s the legal ceiling.
And here’s the irony: The law was designed to help small biotech firms. But big pharma learned how to game it. Instead of relying on one core patent, they file dozens-on formulations, dosages, delivery methods, even metabolites. These aren’t new drugs. They’re tweaks. But they count as new patents. And they keep generics off shelves long after the original patent expires.
Secondary Patents: The Real Secret to Long Exclusivity
Look at any blockbuster drug-Humira, Enbrel, Keytruda-and you’ll see a pattern. The original patent might expire in 2025. But the company has filed 20 or more follow-up patents. One for an extended-release version. One for a new combination with another drug. One for a different injection device. Each one adds another layer of protection.
A 2023 study by the R Street Institute found that high-revenue drugs get 37% more secondary patents than lower-selling ones. For top-selling drugs, the average number of patents per product hits 20 to 30. This is called a “patent thicket.” It’s not about innovation. It’s about delay.
Generic manufacturers can’t enter until every single patent is challenged and invalidated. That takes years. And lawsuits cost millions. Many generics never bother. The brand company wins by default.

Regulatory Exclusivity: The Hidden Clock
Patents aren’t the only barrier. The FDA gives out separate exclusivity periods that run parallel to patents. These don’t depend on patent status. They’re automatic.
- New Chemical Entity (NCE) Exclusivity: 5 years of market protection, no generics allowed.
- New Clinical Investigation Exclusivity: 3 years for new uses or formulations.
- Orphan Drug Exclusivity: 7 years for drugs treating rare diseases.
- Pediatric Exclusivity: 6 months added to any existing exclusivity or patent.
These don’t show up in patent databases. They’re buried in FDA records. But they’re just as powerful. A drug might have its patent expire in 2026, but if it still has orphan drug exclusivity until 2028, generics can’t touch it. Companies time their filings to stack these protections like layers of armor.
Global Differences: It’s Not Just the U.S.
The U.S. isn’t alone. Other countries have similar systems-but with different rules.
Canada offers a Certificate of Supplementary Protection (CSP), giving up to 2 years of extra protection after patent expiry. Japan allows up to 5 years of patent term extension, matching the U.S. But Europe’s system is more restrictive. The Supplementary Protection Certificate (SPC) there caps total exclusivity at 15 years from first marketing authorization.
And in countries without strong patent laws-like India or Brazil-generics arrive faster. That’s why many drugmakers launch new medicines first in the U.S. or EU, where they can maximize profits before generics take over elsewhere.

The .6 Billion Problem
Developing a new drug costs an average of $2.6 billion (in 2013 dollars, adjusted for inflation, it’s over $3.5 billion today). That’s not a guess. It’s from peer-reviewed studies published in the Journal of the American Medical Association and the Tufts Center for the Study of Drug Development.
That kind of investment needs a return. Without exclusivity, no company would risk billions on a drug that might fail in Phase 3 trials. But the system is fraying. The 10- to 15-year window that once felt fair now feels stretched thin by legal maneuvering.
When a drug’s exclusivity ends, prices drop 80% to 90% within a year. A $150,000-a-year cancer drug might fall to $20,000. That’s good for patients. Bad for the company. That’s why pharmaceutical firms spend millions on lifecycle management-extending the life of a drug through minor changes, new indications, or clever marketing.
What’s Next? The Crackdown on Evergreening
Regulators are starting to push back. The FDA has tightened rules on what qualifies for pediatric exclusivity. Courts in the U.S. and EU are invalidating secondary patents that lack real innovation.
In 2024, the U.S. House Energy and Commerce Committee launched an investigation into “patent evergreening.” They’re asking: When does a new tablet form become a new drug? When does a different dosing schedule justify another 5 years of monopoly?
Some experts argue the 14-year cap should be lowered. Others say the system needs a complete reset-maybe replacing patents with prizes or government-funded R&D. But for now, the game continues.
Drugmakers still file patents the moment a molecule is synthesized. They still delay generics with legal tactics. And patients still wait years for affordable versions-even when the science is old.
The effective patent life isn’t broken. It was designed this way. But the balance has tipped. The 20-year patent was never meant to be a 20-year monopoly. It was meant to be a 10-year head start. And for too many drugs, that head start has become a lifetime.
Why does my prescription drug cost so much even after 10 years?
Even after 10 years, the original patent may have expired, but secondary patents-on new formulations, delivery methods, or combinations-can still block generics. Companies often file dozens of these patents to stretch exclusivity. Regulatory exclusivities like orphan drug status or pediatric extensions can also delay generics. So while the core patent is gone, other legal protections keep prices high.
Can generics enter the market as soon as the patent expires?
No. Even after a patent expires, generics can be blocked by regulatory exclusivities (like 5-year NCE protection) or by a 30-month litigation stay. If the brand company sues a generic maker within 45 days of receiving notice, the FDA can’t approve the generic for up to 30 months-unless a court rules in the generic’s favor sooner. This delay is built into the system.
What’s the difference between a patent and regulatory exclusivity?
A patent protects the invention itself-like the chemical structure or method of making the drug. It’s issued by the Patent Office and can be challenged in court. Regulatory exclusivity is granted by the FDA and protects the drug from competition based on its approval timeline, not its invention. It doesn’t require a lawsuit. It just waits out the clock. One can expire while the other is still active.
Why do some drugs have 20+ patents?
It’s called patent thickets. Companies file patents not just on the drug itself, but on every possible variation: extended-release pills, new dosing schedules, different delivery devices, combinations with other drugs, even the color of the pill. These aren’t always new treatments-they’re minor changes. But under current law, each one can block generics. High-revenue drugs get more patents because the financial payoff is worth the legal cost.
Is there a limit to how long a drug can be protected?
Legally, yes. In the U.S., total market exclusivity from FDA approval can’t exceed 14 years under patent term extension rules. But in practice, it often lasts longer because regulatory exclusivities (like orphan drug status) and secondary patents can stack on top. Some drugs maintain de facto exclusivity for 18 to 20 years through this combination. There’s no single cap on all protections combined.


king tekken 6
28 Nov 2025 at 02:57man i always thought patents were 20 years of pure cash... turns out its more like 7 years of screaming into the void while lawyers sip coffee and count bills. the system is rigged and we all pay for it.
and dont even get me started on how they tweak the pill color to reset the clock. jesus.