When you pick up a prescription, you might see a pill that looks exactly like your brand-name medication-but without the brand name on it. That’s not a mistake. It’s an authorized generic. And while it’s sold as a generic, it’s actually made by the same company that made the original brand-name drug. This isn’t just a cheaper version-it’s the exact same drug, down to the inactive ingredients, packaging, and manufacturing process. But here’s the twist: it’s not listed in the FDA’s official generic drug database. So what’s really going on?
What Exactly Is an Authorized Generic?
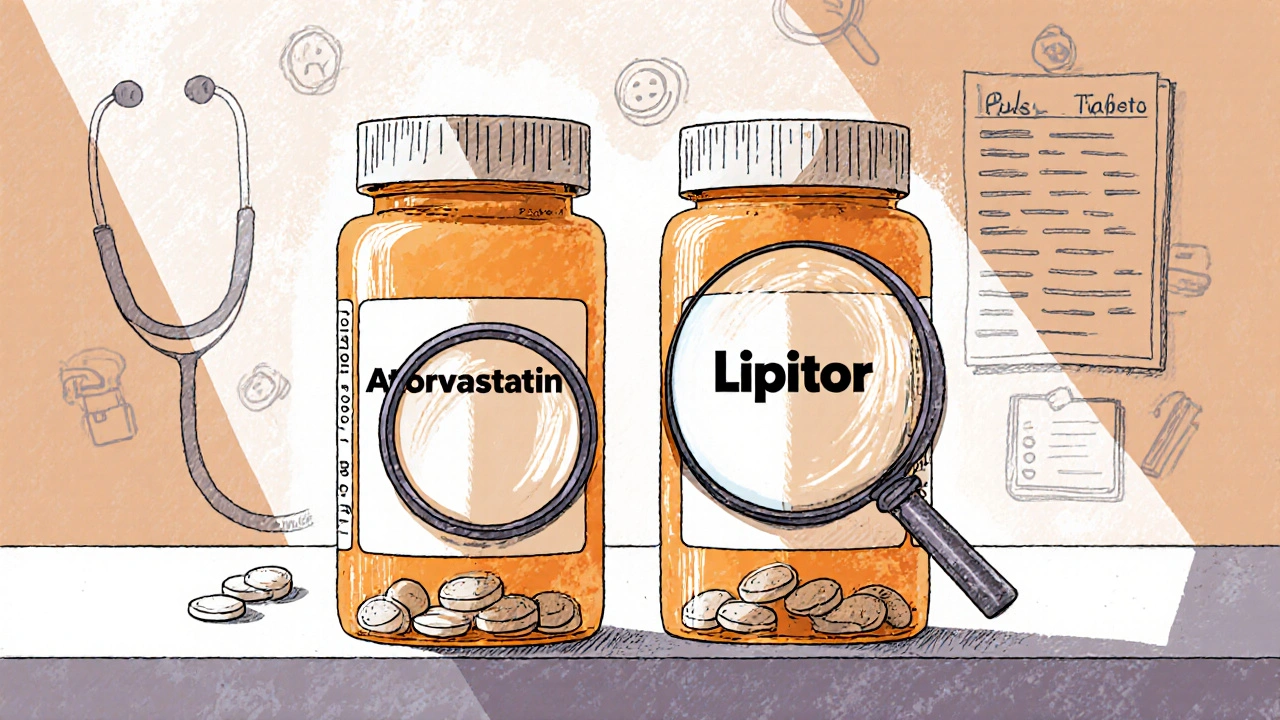
An authorized generic is a brand-name drug sold without the brand name on the label. According to the U.S. Food and Drug Administration (FDA), it’s identical to the brand-name version in every way: same active ingredient, same inactive ingredients, same dosage form, same strength, same shape, same color, same manufacturing site. The only difference? The label doesn’t say "Lipitor" or "Concerta"-it just says "atorvastatin" or "methylphenidate ER."
Unlike traditional generics, which go through the Abbreviated New Drug Application (ANDA) process to prove they’re bioequivalent to the brand, authorized generics don’t need that step. They’re made under the original brand’s New Drug Application (NDA). That means the FDA already approved them when the brand was first launched. All the manufacturer has to do is notify the FDA they’re selling it under a different label.
Think of it this way: if you bought a Coca-Cola and the label was removed and replaced with "Soda, 12 oz, cola flavor," it would still be Coca-Cola. That’s an authorized generic. It’s not a copy. It’s the real thing, just unlabeled.
How Are Authorized Generics Different From Regular Generics?
Regular generics are made by other companies after the brand’s patent expires. They must contain the same active ingredient and meet FDA standards for bioequivalence-but they’re allowed to use different inactive ingredients. That’s why some people notice a change in how a generic pill tastes, how fast it dissolves, or even how it affects their body. A 2023 Pharmacy Times report found that about 15% of patients report noticeable differences when switching from brand to traditional generic, often due to fillers, dyes, or binders.
Authorized generics don’t have that issue. Because they’re made by the brand manufacturer using the exact same formula, they’re physically and chemically identical. No surprises. No formulation tweaks. Just the same drug, same experience, lower price.
Here’s another key difference: traditional generics show up in the FDA’s "Orange Book," the official list of approved generic drugs. Authorized generics? They’re not listed there. You won’t find them in most pharmacy databases unless you specifically look for them. That’s why pharmacists and doctors sometimes don’t realize they’re dispensing one-until the patient asks why their pill looks the same but costs less.
Who Makes Authorized Generics?
There are two ways authorized generics get made:
- The brand-name company makes it themselves and sells it under a different label. For example, Pfizer might make an authorized generic of Lipitor and sell it under the name "atorvastatin" through their own distribution arm.
- The brand licenses the formula to another manufacturer who produces it under contract. This is common when the brand wants to avoid the logistics of selling directly. For instance, Teva or Prasco might produce an authorized generic of a drug like Colcrys (colchicine) on behalf of the original maker.
Either way, the drug comes from the same factory, the same batch process, the same quality control. That’s why authorized generics are often preferred by patients who had bad experiences with traditional generics-especially those with sensitive digestive systems, allergies to certain dyes, or chronic conditions where consistency matters.

Why Do Companies Make Authorized Generics?
It sounds strange: why would a company that just spent billions developing a drug turn around and sell a cheaper version of it? The answer isn’t altruism-it’s strategy.
When a brand’s patent expires, generic competitors flood the market. Prices drop fast. The brand loses market share. But if the brand launches its own authorized generic at the same time, it can keep selling the drug at a lower price and still capture a big chunk of the market. A 2022 Health Affairs study found that between 2010 and 2019, there were 854 authorized generic launches. In 75% of cases, they were introduced after traditional generics had already entered the market-meaning they were a defensive move.
It’s like a chess move. The brand lets one generic in, then drops its own version to undercut the competition. In markets where a single generic company has 180 days of exclusivity (a rule under the Hatch-Waxman Act), the brand often launches its authorized generic during that window to steal sales before other generics arrive.
Result? The brand keeps revenue flowing, patients get lower prices, and the traditional generic companies get squeezed. Critics say this undermines the spirit of the generic drug system. Supporters say it gives patients more affordable options faster.
How Much Do Authorized Generics Cost?
Authorized generics usually cost less than the brand-name drug-but more than traditional generics, especially once multiple generic makers enter the market.
On average, authorized generics are priced 15% to 25% lower than the brand. That’s a big savings. For a drug like Adderall XR, which can cost $300 a month as a brand, the authorized generic might run $200-$250. A traditional generic? Sometimes under $10.
But here’s the catch: authorized generics often come out early. When the first generic hits, it might be priced at $20. A few months later, when five more generics enter, the price drops to $8. The authorized generic? It stays at $20. So if you wait, you might save more. But if you need stability-especially if you’ve had bad reactions to other generics-the authorized version might be worth the extra few dollars.
Some insurers and pharmacy benefit managers (PBMs) prefer authorized generics because they’re predictable. No formulation changes. No complaints from patients. They’re easier to manage in large health systems.
What Are Some Examples?
You’ve probably taken one without knowing it. Here are a few common ones:
- Colchicine (authorized generic of Colcrys) - Used for gout
- Methylphenidate ER (authorized generic of Concerta) - For ADHD
- Celecoxib (authorized generic of Celebrex) - For arthritis
- Levothyroxine (authorized generic of Unithroid) - For hypothyroidism
- Atorvastatin (authorized generic of Lipitor) - For high cholesterol
These aren’t obscure drugs. They’re among the most prescribed in the U.S. If you’re on any of them, ask your pharmacist: "Is this an authorized generic?" You might be paying more than you need to-or you might be getting the exact same drug you were on, just cheaper.

What Should Patients Know?
Many patients don’t realize they’re getting an authorized generic. The pill looks the same. The bottle looks the same. The only clue is the name on the label.
Here’s what you should do:
- Check your prescription label. If the drug name matches your brand but the company name is different, it might be an authorized generic.
- Ask your pharmacist: "Is this the same as the brand?" They can tell you if it’s an authorized generic or a traditional one.
- If you’ve had issues with other generics-like stomach upset, headaches, or inconsistent effects-ask for the authorized version. It’s more likely to work the same way as your brand.
- Compare prices. Sometimes the authorized generic is cheaper than the brand but not the cheapest option. Don’t assume it’s the best deal.
Some patients feel uneasy when they find out their brand-name drug is being sold as a "generic." They worry it’s a trick. But it’s not. It’s the same medicine. The only difference is who’s selling it and how much they’re charging.
Why Aren’t Authorized Generics Listed in the Orange Book?
The FDA’s Orange Book is the official guide to generic drug equivalence. But authorized generics aren’t in it-because they’re not approved as generics. They’re approved as the brand. The FDA treats them as the same product, just rebranded. That’s why they’re not subject to the same rules as traditional generics.
This creates a gray area. Regulators don’t regulate them as generics. Pharmacists don’t track them as generics. Patients don’t know they exist. But they’re a real and growing part of the drug market.
The FDA does maintain a separate, unofficial list of authorized generics. It’s updated periodically, but it’s not easy to find. Most people-including many doctors-don’t know it exists.
Is This Good or Bad for Patients?
It’s both.
On one hand, authorized generics give patients access to the exact same drug at a lower price. For people who can’t tolerate changes in inactive ingredients, they’re a lifeline. For those on long-term meds like thyroid hormone or epilepsy drugs, consistency matters.
On the other hand, they delay true competition. When a brand launches its own generic, it slows down the price drop that happens when multiple companies start making the drug. That means patients might pay more for longer than they would if the market were fully open.
It’s a business move disguised as a benefit. And like most things in pharmaceuticals, it’s complicated.
But here’s the bottom line: if you’re paying full price for a brand-name drug and there’s an authorized generic available, you’re probably overpaying. Ask your pharmacist. Check your bill. You might be saving hundreds a year without changing a single thing about your treatment.





Sullivan Lauer
29 Nov 2025 at 16:33Okay so I just found out my dad’s been taking the authorized generic of Lipitor for three years and he never told me? Like, he’s been saving like $200 a month and I thought he was just being cheap. Turns out he’s not cheap-he’s a pharmaceutical genius. I mean, same factory, same pill, same chemistry, just no fancy branding. It’s like buying the exact same burger but without the golden arches on the wrapper. And guess what? His cholesterol is better than ever. Why are we still paying brand prices when the exact same thing is sitting right there at half the cost? The system is rigged to keep us in the dark, and honestly, I’m mad I didn’t know this sooner. This isn’t just about money-it’s about transparency. We deserve to know what we’re actually getting.
Also, I just called my pharmacist and asked if my Adderall XR was an authorized generic. She paused, looked at the bottle, and said, ‘Ohhhhh, yeah, that’s one.’ I almost cried. I’ve been terrified of generics because of side effects, but this? This is just the real thing with a discount tag. Mind blown.