When you walk into a pharmacy and see two pills that look almost identical-one labeled with a big brand name, the other with a plain chemical name-do you know which one saves you money? Most people don’t. But the numbers don’t lie: generic drugs are saving Americans billions every year, and the gap between what you pay for a brand-name pill versus a generic is wider than most realize.
How Much Do Generic Drugs Actually Save?
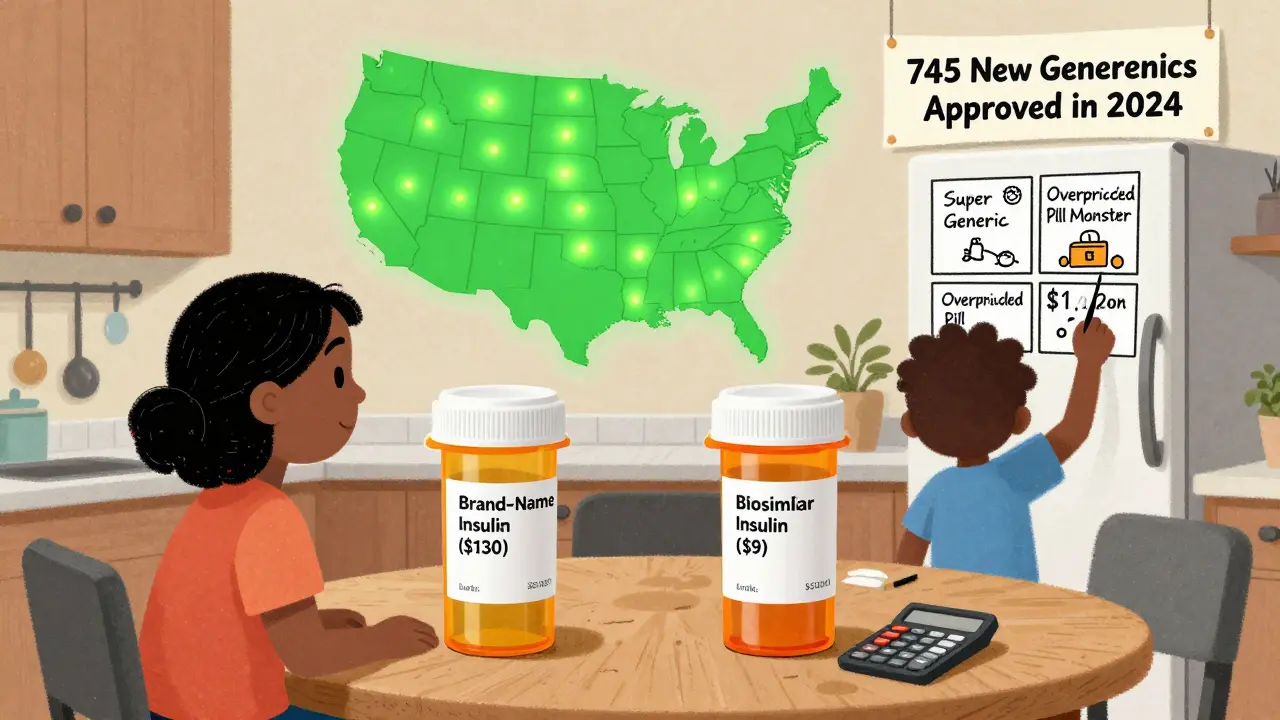
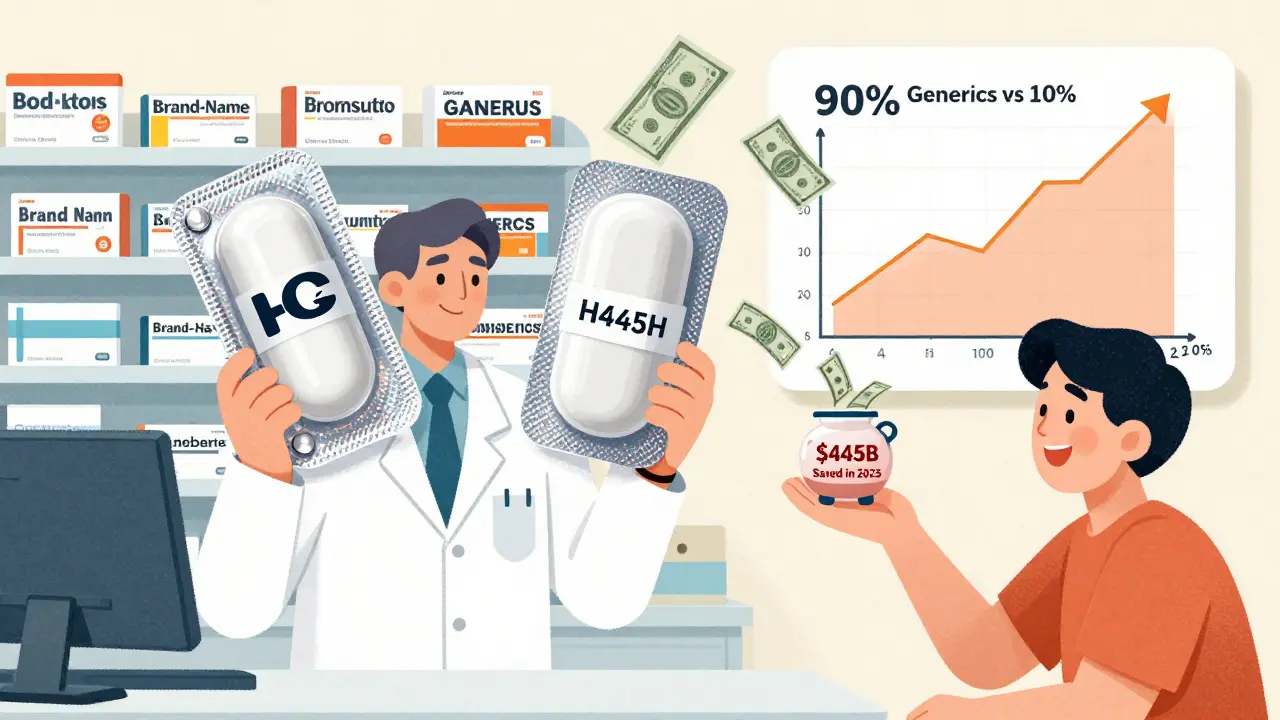
In 2024, Americans filled 3.9 billion prescriptions for generic drugs. That’s 90% of all prescriptions written. Yet those same generics made up just 12% of total prescription drug spending. Meanwhile, brand-name drugs, which accounted for only 10% of prescriptions, sucked up 88% of the money spent on medications.
The math is brutal. The average out-of-pocket cost for a generic prescription in 2024 was $6.95. For a brand-name drug? $28.69. That’s more than four times as much. For uninsured patients, the difference is even starker: brand-name drugs cost an average of $130.18 per prescription-up 50% since 2019-while generic prices dropped by 6% over the same period.
Over the last decade, generic and biosimilar drugs saved U.S. patients and the healthcare system a total of $3.4 trillion. In 2023 alone, that number hit $445 billion. That’s not a guess. It’s from the Association for Accessible Medicines, the industry group that tracks this data. Think about it: $445 billion is more than the entire annual budget of the U.S. Department of Education. And it all came from pills that work exactly the same as their expensive brand-name counterparts.
Why Are Generics So Much Cheaper?
It’s not magic. It’s the law. The Hatch-Waxman Act of 1984 created a shortcut for generic manufacturers. Instead of running expensive clinical trials again, they just had to prove their drug was bioequivalent to the brand-name version-same active ingredient, same strength, same way it’s taken. That cut development costs by up to 80%.
Once a brand-name drug’s patent expires, multiple generic companies can jump in. Competition drives prices down. For example, when the cholesterol drug Lipitor lost its patent in 2011, over 30 generic makers entered the market. The price per pill dropped from $4.50 to less than $0.10. That’s a 98% price collapse.
And it’s still happening. In 2024, the FDA approved 745 new generic drugs. That’s the highest number in over a decade. And it’s not slowing down. Drugs like Entresto (heart failure), Tradjenta (diabetes), and Opsumit (lung disease)-each bringing in over $1 billion in annual sales-are set to lose patent protection in late 2025. When generics hit the market, we could see savings of $8.6 billion in just those three drugs alone.
Biosimilars: The Next Wave of Savings
Not all drugs are simple pills. Some are complex biologics-made from living cells, like insulin, rheumatoid arthritis treatments, and cancer drugs. These used to be impossible to copy. But now, we have biosimilars: near-identical versions that are just as safe and effective.
Since their introduction, biosimilars have saved the U.S. healthcare system $56.2 billion. In 2024, they saved $20.2 billion in one year. For cancer patients, the impact is life-changing. In 2020, biosimilars helped cut the growth of cancer drug spending in half and saved $18 billion on cancer medicines.
One of the biggest wins? Stelara, a drug for psoriasis and Crohn’s disease. When nine biosimilars launched by July 2025, the price dropped by up to 90%. That means patients who once paid $10,000 a year now pay under $1,000. And it’s not just Medicare patients benefiting-commercial insurers and Medicaid are seeing the same drops.

Why Don’t More People Use Generics?
Here’s the problem: it’s not about effectiveness. It’s about access. Many doctors still default to prescribing brand names. Some patients assume generics are “weaker.” Others are just used to the name they’ve always known.
But the real barrier is corporate tactics. Big pharma uses something called “patent thickets.” They file dozens-sometimes over 75-overlapping patents on one drug. This stretches out their monopoly. One drug that should’ve lost its patent in 2016 was protected until 2034 because of this trick.
Then there’s “product hopping.” A company slightly changes a drug’s formulation-say, switching from a pill to a liquid-and then patents the new version. They tell doctors to stop prescribing the old one, even though it’s still perfectly safe. This shuts out generics for years.
Blue Cross Blue Shield estimates these practices cost consumers $3 billion a year. And “pay-for-delay” deals-where brand companies pay generic makers to hold off launching their version-cost the system another $12 billion annually.
What’s Being Done About It?
There’s momentum. In early 2025, bipartisan congressional committees advanced two bills: the Affordable Prescriptions for Patients Act, which targets patent thickets, and the Drug Competition Enhancement Act, which bans product hopping. If passed, these could unlock $3 billion in savings over ten years.
Pharmacy benefit managers (PBMs)-the middlemen who negotiate drug prices for insurers-are also stepping up. As of January 2025, 87% of commercial health plans now require pharmacists to substitute generics when available. Kaiser Permanente cut pharmacy costs by 30% in just 18 months by pushing generics hard.
States are catching up too. Only 42 out of 50 states have modern pharmacy laws that let pharmacists automatically switch to generics unless the doctor says no. The rest still require doctors to write “dispense as written” on every prescription, even when a cheaper, equally effective option exists.
Are Generics Really the Same?
Yes. The FDA requires generics to meet the same strict standards as brand-name drugs. They must have the same active ingredient, same dose, same way they’re taken, and same effect in the body. The FDA tests them. They monitor them. And they track side effects.
Less than 1% of adverse events reported to the FDA since 2019 involved generic drugs. That’s the same rate as brand-name drugs. Some patients report feeling different on a generic from a different manufacturer-but this is rare and usually happens with drugs that have a narrow therapeutic index, like blood thinners or seizure meds. Even then, switching between generics is safe under medical supervision.
The truth? If your doctor says a generic is right for you, it’s not a compromise. It’s a smart choice.
What This Means for You
If you’re paying for prescriptions out of pocket, or even if you have insurance, ask your pharmacist: “Is there a generic version of this?”
Don’t assume your doctor already knows. Ask. It takes 30 seconds. And if your doctor says no, ask why. Is it because the generic won’t work? Or because they’re not updated on the latest options?
For Medicare beneficiaries, the Inflation Reduction Act capped insulin at $35 a month-but that cap doesn’t apply to brand-name insulin. If you’re on insulin, switching to a generic or biosimilar could cut your cost even further.
And if you’re on a chronic condition-diabetes, high blood pressure, depression, asthma-your monthly generic could be saving you hundreds, even thousands, a year. Multiply that by 10 years? That’s a vacation. A car payment. A down payment on something bigger.
The system isn’t perfect. Big pharma still fights hard to protect profits. But the data is clear: generics work. They’re safe. And they’re saving lives by making treatment affordable.
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generic drugs to have the same active ingredients, strength, dosage form, and route of administration as the brand-name version. They must also be bioequivalent-meaning they work the same way in the body. Over 90% of generic drugs are rated as therapeutically equivalent by the FDA. Less than 1% of adverse events linked to generics are due to effectiveness issues.
Why are generic drugs so much cheaper?
Generic manufacturers don’t have to repeat expensive clinical trials. Thanks to the Hatch-Waxman Act of 1984, they only need to prove their drug is bioequivalent to the brand. That cuts development costs dramatically. Once multiple companies start making the same generic, competition drives prices down. For example, after Lipitor’s patent expired, over 30 generic makers entered the market, and the price dropped from $4.50 to under $0.10 per pill.
Do insurance plans cover generic drugs?
Yes. Most insurance plans, including Medicare Part D and Medicaid, encourage or require generic use. Many have lower copays for generics-sometimes as low as $5 or $10-while brand-name drugs can cost $50 or more. In fact, 87% of commercial health plans now require pharmacists to substitute generics when available, unless the doctor specifically says not to.
Can I switch from a brand-name drug to a generic?
In most cases, yes. Talk to your doctor and pharmacist. If your medication has a generic version, switching is safe and often recommended. For most drugs, including blood pressure pills, antidepressants, and statins, there’s no clinical reason to stay on the brand. The only exceptions are drugs with a narrow therapeutic index, like warfarin or levothyroxine, where small differences in absorption matter. Even then, switching between generics is monitored and safe under medical supervision.
What’s the difference between a generic and a biosimilar?
Generics are copies of simple chemical drugs, like pills. Biosimilars are copies of complex biologic drugs made from living cells-like insulin, rheumatoid arthritis treatments, and cancer drugs. Biosimilars aren’t exact copies, but they’re highly similar in structure and function, with no meaningful difference in safety or effectiveness. They’re approved under a different FDA pathway and have saved over $56 billion since their introduction.
Will I save money if I use generics?
Absolutely. In 2024, the average out-of-pocket cost for a generic prescription was $6.95. For a brand-name drug, it was $28.69. That’s more than four times more. For uninsured patients, brand-name drugs cost over $130 per prescription-while generics stayed under $10. Over a year, switching just two medications to generics can save you $800 or more.
What’s Next for Generic Drugs?
The pipeline is full. Over 700 new generic drugs are expected to launch in 2025 and 2026. Biosimilars for major drugs like Humira and Enbrel are on track to hit the market, potentially saving tens of billions more.
The Congressional Budget Office projects that generic and biosimilar competition will keep overall drug spending growth at just 3.2% annually through 2030. Without them, brand-name drug spending would grow at 6.8%-nearly double.
It’s not about choosing between quality and cost. It’s about choosing between paying too much and paying fairly. Generics aren’t a second option. They’re the smart, proven, and powerful tool that keeps healthcare affordable for millions. And if you’re not using them, you’re leaving money on the table-and possibly risking your health by skipping doses because you can’t afford your meds.
Ask for the generic. Ask why not. And don’t let a label tell you what you’re worth.






James Dwyer
28 Jan 2026 at 11:24Just switched my blood pressure med to generic last month. Paid $12 for a 30-day supply. Used to be $87 with insurance. My wallet and my sanity thank you.