When you or someone you love gets a cancer diagnosis, the next question isn’t just cancer clinical trials-it’s: Are they right for me? Many people assume trials are last-resort experiments. That’s not true. They’re the backbone of modern cancer care, and thousands of patients benefit every year-not just by getting new treatments, but by being part of something bigger.
What Are the Phases of a Cancer Clinical Trial?
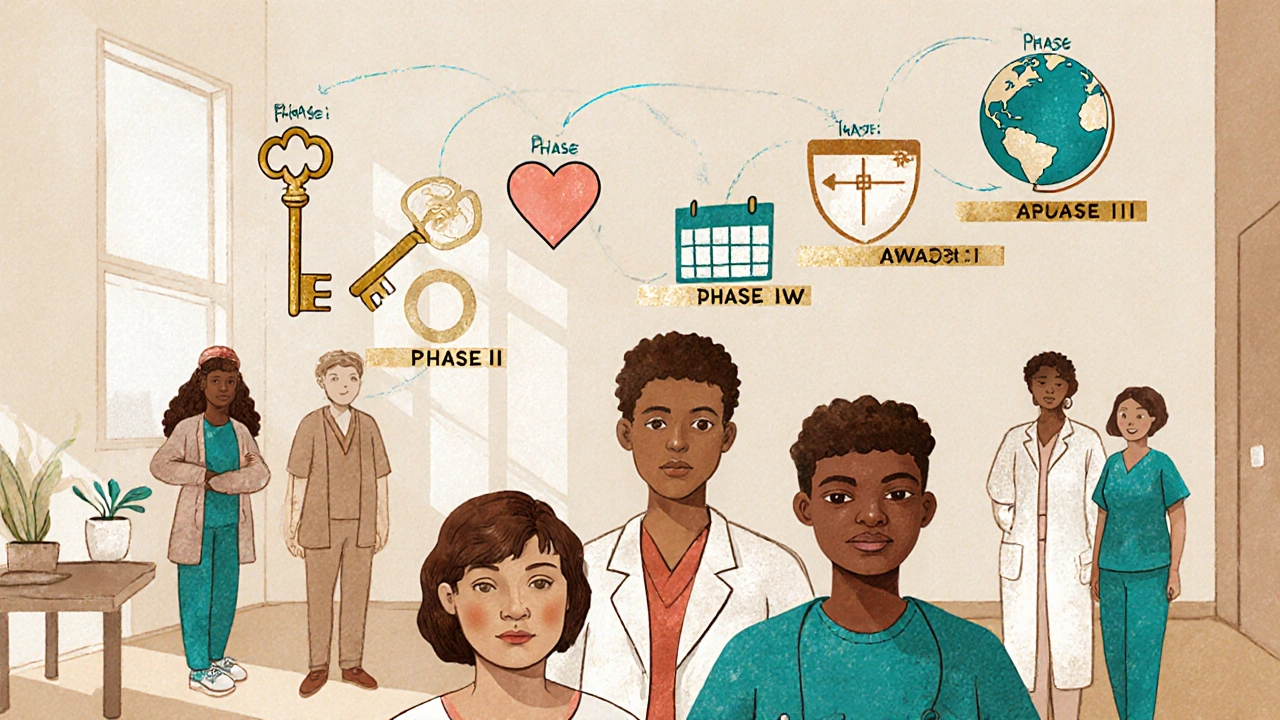
Cancer clinical trials don’t jump straight from the lab to your body. They follow a strict, five-step system built over decades to protect patients while finding real breakthroughs. Each phase answers one specific question, and only if the answer is good enough does the trial move to the next step.Phase 0 is the tiniest step. It involves just 10 to 15 people and uses a dose so low it won’t treat cancer-it’s just to see if the drug even reaches the tumor and how the body breaks it down. Think of it like testing a key in a lock before trying to turn it. This phase helps researchers decide whether a drug is worth studying further.
Phase I is where safety comes first. Around 20 to 80 people join, often those who’ve run out of standard options. The goal? Find the highest dose you can give without causing serious harm. Doctors start with tiny amounts and slowly increase, watching closely for side effects. This phase doesn’t care if the drug shrinks tumors-it cares if it’s safe enough to try on more people. Most drugs fail here. That’s not a bad thing. It means the system is working.
Phase II shifts focus to effectiveness. Now, 25 to 100 people with the same type of cancer get the treatment. Researchers watch: Does it shrink tumors? Slow growth? Improve symptoms? This phase is where you start seeing real hope. A drug might look amazing in a lab, but Phase II tells you if it works in real patients. About half of drugs that reach this stage don’t move forward-because they don’t work well enough, or the side effects are too harsh.
Phase III is the big test. Hundreds to thousands of people join, often across multiple countries. Half get the new treatment. The other half get the current standard care. Neither the patient nor the doctor usually knows who’s getting what-that’s called randomization and blinding. It’s the only way to know for sure if the new treatment is better. These trials take years. If the results show a clear advantage-like longer survival or fewer side effects-the drug can be approved by the FDA.
Phase IV happens after approval. Thousands more patients take the drug in real-world settings. Doctors track long-term side effects, how it works with other medications, and if it helps different groups of people. This phase catches problems that only show up after years of use. It’s how we know if a drug is truly safe over time.
Why Would Someone Join a Cancer Clinical Trial?
People join for many reasons. Some have tried everything else. Others want to be part of the next breakthrough. But the benefits go beyond just access to new drugs.First, you get more attention. Trial participants are monitored more closely than patients on standard care. You’ll have more blood tests, scans, and check-ins. One 2022 survey found 78% of participants felt their care team was more responsive to side effects. That matters when you’re feeling awful.
Second, you might get something that works when nothing else did. A woman with stage 4 melanoma joined a Phase II immunotherapy trial after chemotherapy failed. Three years later, she’s cancer-free. Her story isn’t rare. Thousands like her have lived longer-or even been cured-because of trials.
Third, you help others. Eighty-five percent of participants say knowing they’re helping future patients gives them purpose. That’s not just emotional-it’s real. Every trial, even the ones that fail, teaches scientists something. That knowledge saves lives down the line.
Some people worry they’ll get a placebo. That’s rare in cancer trials. Most Phase III trials compare the new treatment to the current best option, not to nothing. You’re not being left behind-you’re being compared to what’s already out there.
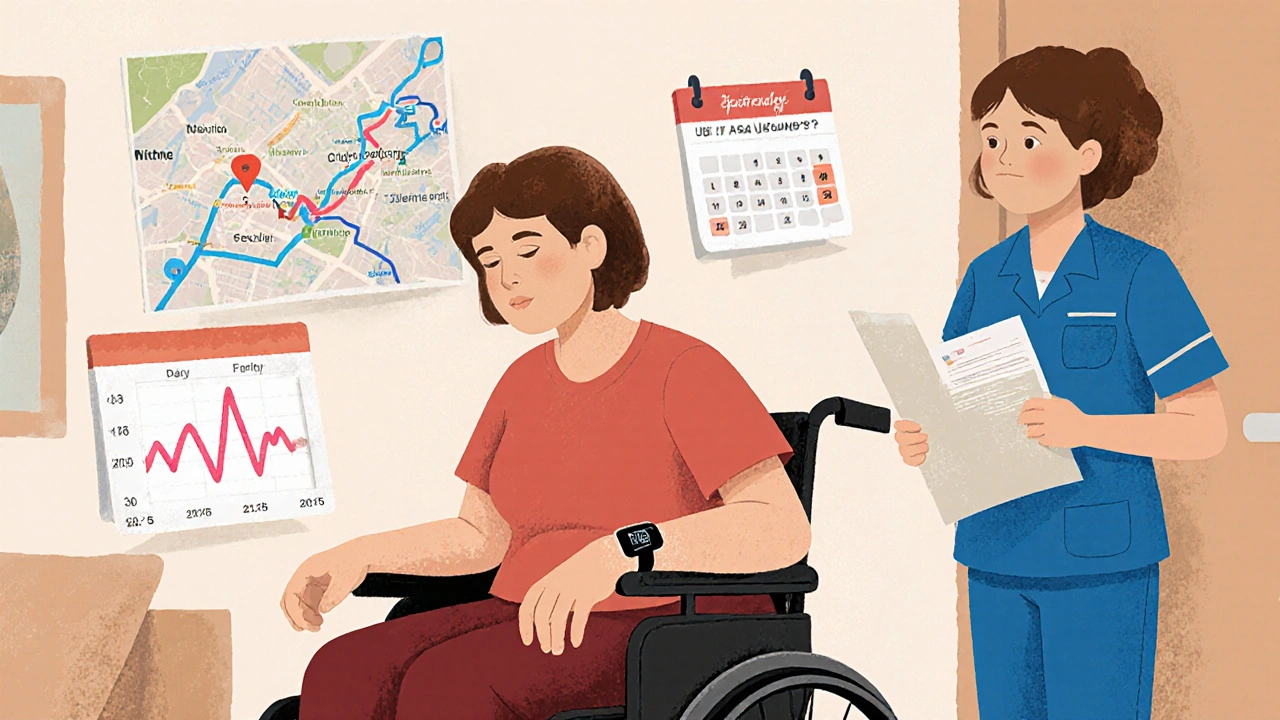
What Are the Real Challenges of Joining?
It’s not all hope and progress. There are real hurdles.Travel is the biggest one. Many trials require visits every week or two. For someone living in a rural area, driving three hours for a scan while feeling sick from treatment isn’t just inconvenient-it’s exhausting. One Reddit user described it as "driving 3 hours each way while barely able to get out of bed." That’s why patient navigators are so important. These are staff members trained to help with transportation, scheduling, and paperwork. They’re available at 78% of top cancer centers.
Eligibility rules are strict. The average trial has 28 criteria for who can join. Age, previous treatments, lab results, even other health conditions can disqualify you. That’s why only 3% to 5% of adult cancer patients in the U.S. join trials-even though experts say up to 20% could be eligible. It’s not that people don’t want to. It’s that the system makes it hard.
Randomization can be scary. If you’re in a Phase III trial, you might get the standard treatment instead of the new one. That’s hard to accept. But remember: the standard treatment is what’s already proven to work. You’re not getting less care-you’re helping prove if something better exists.
And then there’s the time. A Phase III trial can last 1 to 4 years. That’s a long commitment when you’re already fighting cancer. But many participants say the structure helps. Knowing what to expect each week gives them a sense of control.
How Are Trials Changing Today?
The old system isn’t broken-it’s just being upgraded.Instead of testing one drug on one cancer type, researchers now run basket trials and umbrella trials. A basket trial tests one drug on many cancers that share a genetic mutation. An umbrella trial tests many drugs on one cancer type, matching each drug to a specific mutation. This is precision medicine in action.
More trials are using wearables. Sensors track heart rate, sleep, activity levels-even when you’re at home. That means fewer trips to the hospital and more real-life data.
There’s also a push to include more diverse patients. Right now, only 8% of trial participants are Black, even though Black people make up 13% of cancer deaths. New programs are working to fix that, with outreach in communities that have been left out.
And thanks to the FDA’s Project Facilitate, patients with no other options can get access to experimental drugs faster-over 1,200 have already benefited.

What Should You Do If You’re Considering a Trial?
Start by talking to your oncologist. Ask: "Are there any trials I might qualify for?" Don’t wait until you’ve run out of options. Trials can be an option early on.Use trusted sources. The National Cancer Institute (NCI) has a free database of active trials at cancer.gov/clinicaltrials. You can search by cancer type, location, and drug.
Ask questions. What phase is it? What’s the goal? What are the risks? Will I know which treatment I’m getting? What if I want to quit? There’s no such thing as a dumb question. Research coordinators are there to help you understand.
Bring someone with you. Trials are complex. Having another set of ears helps you remember what was said.
And remember: you can leave at any time. No one can force you to stay. Your safety and comfort come first.
Is It Worth It?
There’s no easy answer. But for many, the answer is yes.Clinical trials aren’t magic. Most drugs fail. Most people don’t join. But the ones who do? They’re not just patients. They’re pioneers. They’re the reason we have targeted therapies, immunotherapies, and better survival rates today.
Every drug approved in the last 20 years went through this process. Every new hope-every chance to live longer, better, with fewer side effects-started in a trial. And if you join, you’re not just getting care. You’re helping build the next generation of care.
The system isn’t perfect. It’s slow. It’s complicated. But it’s the best we have-and it’s saving lives every day.
Are cancer clinical trials safe?
Yes, but safety is built in step by step. Phase I trials test the highest risk treatments on the smallest groups, starting with very low doses. Every step is reviewed by ethics boards and government agencies. You’ll be monitored closely, and you can leave anytime. The system exists to protect you-not to experiment on you.
Do I have to pay to join a clinical trial?
Usually not. The trial sponsor covers the cost of the experimental treatment, extra tests, and monitoring. Your regular care-like standard chemotherapy, blood work, or scans-is typically billed to your insurance. Some trials even cover travel or lodging. Always ask for a clear breakdown before signing up.
Can I join a trial if I’ve already had treatment?
Yes, many trials are designed for people who’ve tried standard treatments and need something new. Some trials even look for patients who haven’t had any treatment yet. It depends on the trial’s goals. Your medical history helps determine which trials you’re eligible for-not disqualifies you entirely.
What if I’m not eligible for any trials?
That’s more common than you think. About 80% of cancer patients don’t qualify for trials due to strict rules. But that doesn’t mean there’s nothing left. Talk to your doctor about other options: compassionate use programs, expanded access, or second opinions at major cancer centers. New trials open every week-ask to be notified if one matches your profile later.
How long does it take to get into a trial?
It usually takes 10 to 14 days from your first inquiry to enrollment. That’s because you need medical records, scans, lab tests, and a review by the trial team. Some trials move faster if you’re already at the center. Others take longer if they need more data. Be patient, but keep asking. Your persistence matters.
Can I switch from a trial to standard care if it’s not working?
Absolutely. You can withdraw from a trial at any time, for any reason. If the treatment isn’t helping or side effects are too much, your care team will help you transition back to standard options. Your health comes first-always.



Kelsey Robertson
17 Nov 2025 at 17:30Let me just say this: if you're joining a trial because you 'want to help future patients,' you're either delusional or a saint. Most people don't even know what phase they're in. They just sign the 47-page consent form because the doctor said 'it's your last hope.' And then they die in a hospital 200 miles from home, surrounded by strangers who call them 'a valuable contributor.'
Yes, trials are 'the backbone of modern cancer care'-but only if you're a shareholder in Big Pharma. The real backbone? The $2 billion in taxpayer money that funds the research so a CEO can get a yacht.
And don't get me started on 'randomization.' You think it's fair? What if you're the one who gets the placebo? Oh wait-you don't get a placebo. You get the drug that failed Phase I because it turned people into screaming zombies. But hey, at least you're 'helping science.'
Also, 'patient navigators'? That's just a fancy word for someone who gets paid to tell you, 'Sorry, your insurance won't cover the Uber to the trial site.' You're supposed to drive three hours? Good luck with that. I've seen people show up in wheelchairs, crying, because their child had to miss school to drive them. This isn't progress. It's exploitation dressed up in clinical jargon.
And don't even mention 'diversity.' They want more Black participants? Cool. How about you stop making trials require 28 eligibility criteria that only rich, white, suburban patients can meet? Like, maybe don't disqualify people because they have hypertension? Oh wait-you do. Because your 'precision medicine' only works on people who've never missed a meal or missed a doctor's appointment.
It's not about helping. It's about control. And the system knows it. That's why they never talk about Phase IV-because that's when the real damage shows up. And by then? You're dead. And they've already patented the next drug.
I'm not saying don't join. I'm saying: know what you're signing up for. You're not a pioneer. You're a data point. And the only thing you're guaranteed? A 90% chance of being ignored after you're done.