JAK Inhibitor Risk Assessment Tool
Personal Risk Assessment
This tool helps evaluate your individual risk for infections and blood clots when taking JAK inhibitors based on medical guidelines.
Risk Assessment Results
Infection Risk
Blood Clot Risk
Important Medical Guidance
These results are for educational purposes only. Consult your rheumatologist before making treatment decisions.
When you start a JAK inhibitor for rheumatoid arthritis, psoriasis, or another autoimmune condition, you’re not just getting relief from joint pain or skin flare-ups. You’re also stepping into a world where your body’s natural defenses are quietly turned down - and that comes with real, measurable risks. Two dangers stand out above all others: infections and blood clots. These aren’t rare side effects you read about in fine print. They’re serious, life-altering events that doctors now screen for, monitor closely, and sometimes prevent entirely - if you catch them early.
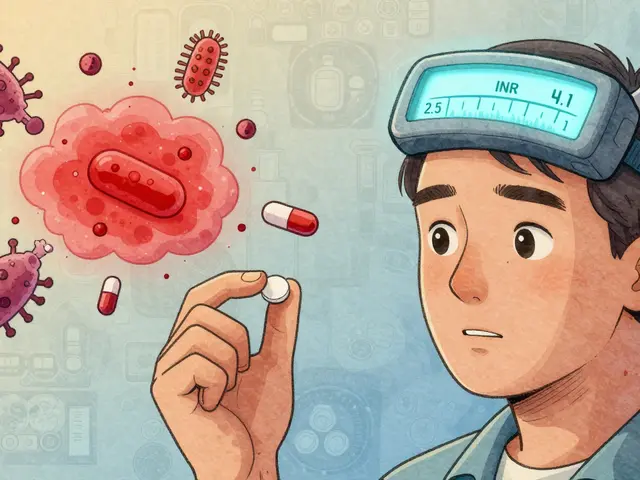
Why JAK Inhibitors Increase Infection Risk
JAK inhibitors work by blocking signals inside immune cells that tell the body to attack itself. That’s great for calming down inflammation. But those same signals also help your body fight off bacteria, viruses, and fungi. When you suppress them, your defenses get sluggish. The most common serious infection linked to these drugs is herpes zoster - better known as shingles. Studies show about 1 in 7 people taking a JAK inhibitor develop shingles within the first year, even if they’ve had the vaccine. That’s more than double the rate seen in people on older biologics. The virus reactivates because your immune system can’t keep it in check anymore. Other infections to watch for include tuberculosis (TB), pneumonia, and fungal infections like candidiasis. People on JAK inhibitors are more likely to get these infections, and they’re more likely to get them badly. One patient in Leeds reported being hospitalized after a mild cough turned into bacterial pneumonia within weeks of starting upadacitinib. Her doctor later said she’d likely had undiagnosed latent TB that flared up because the drug suppressed her immune response. The FDA and EMA now require black box warnings for serious infections. That means your doctor should be asking you: Have you ever had TB? Have you traveled to areas where fungal infections are common? Are you up to date on vaccines? If not, they shouldn’t start you on a JAK inhibitor until those questions are answered.Thrombosis: The Silent Threat
While infections are obvious, blood clots sneak up on you. Venous thromboembolism (VTE) - which includes deep vein thrombosis (DVT) and pulmonary embolism (PE) - is one of the most dangerous side effects of JAK inhibitors. And it’s not just a theoretical risk. Data from the ORAL Surveillance trial showed that patients on tofacitinib had more than double the risk of pulmonary embolism compared to those on TNF inhibitors. Here’s what you need to know: JAK2 inhibition affects platelet production and blood clotting factors. That doesn’t mean your blood thickens - it means your body’s natural balance tips toward clotting. The risk isn’t the same for everyone. If you’re over 65, smoke, are overweight (BMI over 30), have a history of clots, or are on estrogen therapy, your risk jumps significantly. One 2023 analysis found that patients with prior VTE had more than five times the risk of another clot on a JAK inhibitor. Real-world stories back this up. A Reddit user from Manchester described waking up with severe calf pain six months after starting upadacitinib. An ultrasound confirmed a deep vein thrombosis. Her rheumatologist stopped the drug immediately. She’s now on anticoagulants and switched to a different class of medication. Even if you feel fine, you can still be developing a clot. Symptoms are often subtle: swelling in one leg, unexplained shortness of breath, chest pain that gets worse when you breathe. If you’re on a JAK inhibitor and notice any of these, don’t wait. Go to urgent care. Don’t assume it’s just muscle soreness or a bad flight.Who Should Avoid JAK Inhibitors Altogether?
Not everyone is a candidate. Regulatory agencies have tightened restrictions because the risks aren’t evenly distributed. The European Medicines Agency (EMA) and FDA now say JAK inhibitors should only be used if:- You’ve tried and failed other treatments like TNF inhibitors
- You’re under 65
- You don’t smoke
- You have no history of blood clots, heart attack, or stroke
- You don’t have active cancer or a recent cancer diagnosis
- Your BMI is below 30

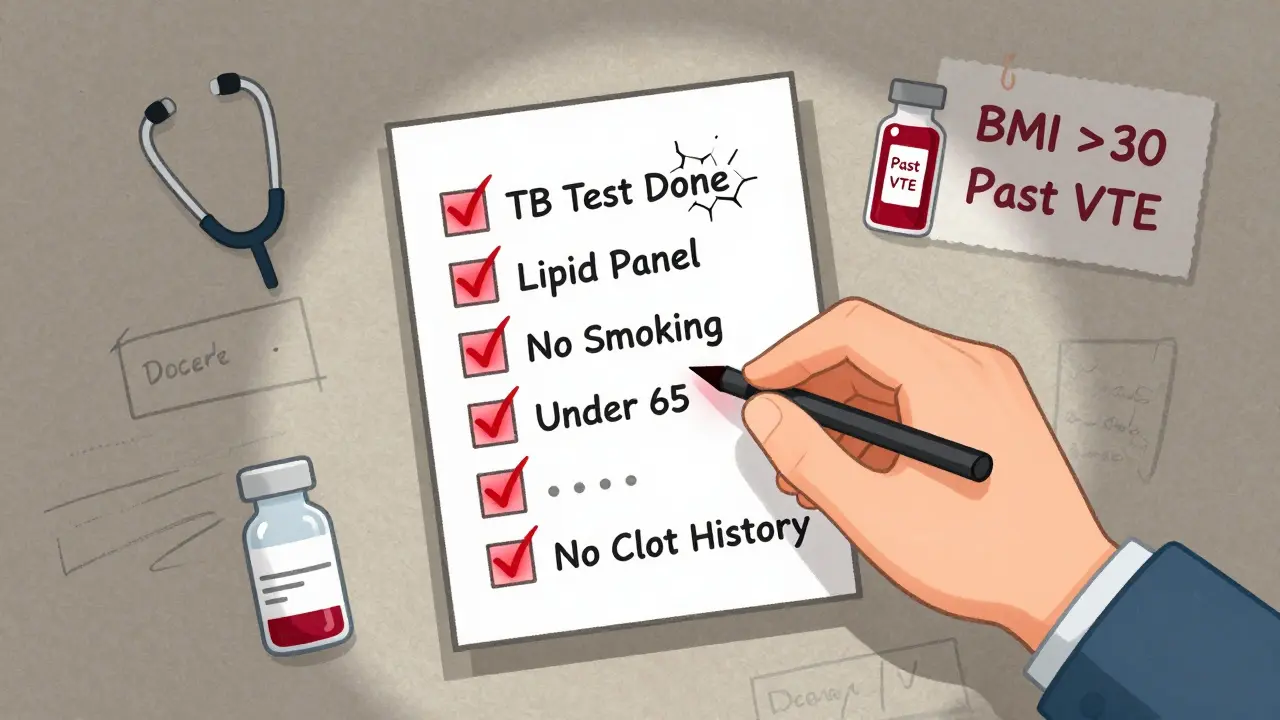
What Your Doctor Should Check Before You Start
Before prescribing a JAK inhibitor, your rheumatologist should do more than just sign a form. They need to build a full picture of your health. Here’s what a responsible provider checks:- History of blood clots - even if it was years ago
- Cardiovascular risk factors - high blood pressure, diabetes, high cholesterol
- Smoking status - current or past
- Age - risk increases sharply after 65
- Cancer history - including skin cancer
- Vaccination status - especially for shingles, pneumonia, and flu
- Baseline blood tests - CBC, lipid panel, D-dimer (in high-risk patients)
Monitoring After You Start
Starting a JAK inhibitor isn’t a one-time decision. It’s an ongoing commitment to monitoring. Here’s what you’ll need to do:- Every 4-8 weeks: Complete blood count to check for low white cells, red cells, or platelets
- At 4 and 12 weeks: Lipid panel to track cholesterol changes
- Every 3 months: Review for signs of infection - fever, cough, skin rashes, urinary symptoms
- Immediately if symptoms appear: Swelling, pain, or redness in one leg; sudden shortness of breath; chest pain






Priyanka Kumari
15 Jan 2026 at 08:06Just wanted to say this is one of the clearest, most responsible summaries of JAK inhibitor risks I’ve seen. As someone managing RA for 8 years, I’ve seen too many people get rushed into these meds without proper screening. The TB and VTE warnings? Non-negotiable. If your doc skips the IGRA test or lipid panel, find a new one. This isn’t just medical advice-it’s survival guidance.